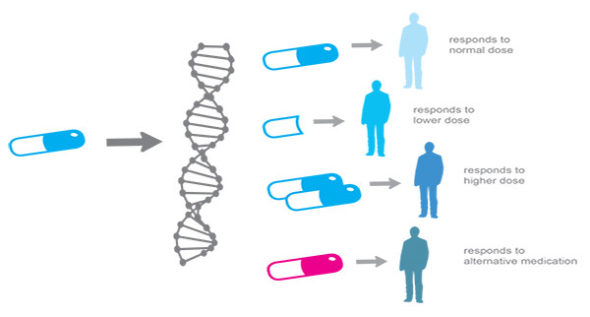
How tumor microenvironment shapes personalized medicine
Personalized medicine was first described in 2005 when cancer therapy has been shown to be well-defined in subgroups of patients having the same tumor. This had led to extensive research where it turned out to be that patients had the same cancer phenotype differ in their disease molecular subtypes with significantly different features and significant response/ resistance to cancer therapy. This discovery has revolutionized cancer therapeutic research and scientists started to appreciate tumor heterogeneity first in 2008. Thereafter, cancers have been reclassified based on their heterogeneity to identify the most suitable treatment options for patients. I will sort here a few examples before I go deeply in the rationale behind these classifications. To the best of our knowledge, we can divide breast cancer based on its molecular content into four subtypes: luminal A (Estrogen Receptor (ER)+, Progestogen Receptor (PR)+, HER2 and Ki67 <14%), luminal B (ER+, PR+, HER2 and Ki67 >14% or ER+, PR+, HER2+), HER2 positive breast cancer (HER2+, ER and PR) and basal-like (ER, PR and HER2). Colon cancer also can be divided into RAS/BRAF-positive or negative or types of epigenetic changes CIMP-positive/ negative or microsatellite instability profile MSI-high, low or stable. The classification of the aforementioned tumor subtypes is indeed becoming detrimental when it comes to the selected cancer treatment regimen. It was not until 2009 where tumor microenvironment pulled the attention of the scientific community towards the development of significant achievements in cancer therapy based on the modulation of the tumor surrounding components. The interaction between tumor networks, which consist of; malignant cells, fibroblasts, immune cells, blood vessels, lymphatics, small signaling and inflammatory molecules such as chemokines, cytokines, growth factors, secretory proteins and degrading enzymes that regulate wide varieties of cellular functions, has led to what we refer today as tumor microenvironment.
With the availability of different treatment modalities, we must think about the core content of the cancer tissue from the following aspects:
1. The ratio of inflammatory molecules or cells to each other in the cancerous tissue
Type and number of infiltrating leukocytes can be crucial for cancer survival. The predominant colocalization of neutrophils in tumor tissue has been shown to boost tumor progression, and high neutrophils to lymphocyte ratio have been well correlated with poor patient’s prognosis. Moreover, tumors with a solid signature of macrophages infiltration have shown strong resistance for many therapeutic modules. Cancer cells can educate macrophages very well and turn them into what so-called M2 macrophages. M2 macrophages not only provide cancer some of the critical survival factors such as VEGF or cytokines but also augment cancer metastasis by releasing the required matrix-degrading enzymes, for instance, MMP2 and MMP9. On the other hand, cytokines and chemokines produced by stroma can have a major impact on tumorigenesis, for example; an increased ratio of CCL17/CCL22 has been correlated with poor outcomes in colorectal cancer. Not very far, lymphocytes are always there which can complicate the scenario even further. Regulatory lymphocytes Tregs are well documented to be one of the most important factors that favored cancer survival.
2. The signaling pathways and cancer therapy resistance
Cancer researchers have uncovered fascinating ways to modulate cancer microenvironment. The molecular map of each cancer patient is indeed different and mapping each patient for diagnostic or prognostic purposes becomes feasibly unreasonable. Ideally, if we are looking for an effective and selective treatment, we need to dissect to the ground every single information of each patient’s tumor tissue. This is literally impossible at least currently. What we need is to identify biomarkers. Biomarkers for diagnosis and prognosis are quite convinceable to deal with both within cost aspects and application perspectives. However, the massive number of signaling pathways and malignant transformation becomes indeed red traffic that we need to stop in front for a while. The aberrant molecular pathways in different cancers have created dilemmas to selectively target these pathways. Firstly, switching one molecular target off might activate the non-canonical pathway of other oncogenes/tumor suppressor genes or harms untransformed cells. Secondly, the complexity of the tumor microenvironment is another challenge. Cancer is in continuous communication with its surroundings and in fact, cancer exploits the ultimate benefits from this interaction to further progress and keep factors of survival alternatives available. For example; under stress conditions, cancer secretes HIF which upregulates VEGF that is required for angiogenesis from the surrounding stroma. Therefore, cancer microenvironment orchestrates different signaling pathways according to the cancer demands hence the need to target different pathways at the same time would have another negative impact on healthy cells. Thirdly, cancer resistance to treatment or DNA mutation in response to treatment is also common. One major challenge is cancer stem cells. Cancer stem cells utilize MDR protein mutations and ABC transporters which enhance drug efflux creating significant barriers for drug delivery. Another challenge is created by the chemotherapeutic drugs themselves. Chemotherapy is usually targeting happy proliferating cancer cells while leaving behind dormant cancer stem cells in the quiescent phase, therefore the need for drugs targeting this stage is greatly needed.
it is no doubt that personalized medicine will take cancer therapeutics into a totally another paradigm, however, deep understanding of tumor microenvironment is still at its infancy and requires fundamental research work.
9s44qtqQCu5
V33TJi7psnH
HGMAmKLdxP0
Kv0NSkpGFQF
6QmIYBQO2HW
nnap7IyOGQi
8JHv4AXE4mt
1KfmUDzRXds
jukLR5tIA1k
bRq2kHvPpEr
Ls4xsa6teha
WQNSVFVu4f8
Zqf1sbstlld
mOKAUbk419m
FygA3vZDQDg
qPNANjGBkFz
aQxWgRQXzVi
go8jU6fggQ2
nkyG5m0ghRY
6EjthvFm548
KBdfy3qNoLz
0CmhBpl0UTL
H8iKp83bOvm
1TO7TAiyvCs
7ku2Zyacsad
cAKiAIOZ76j
KAL4KB34swS
qxRg2uKJBUi
cTvNDIkxrub
KG0ASXeDNOy
hB0CTISGXDr
yo5AdHeI71g
ZtqyZpYn0EY
fUBWxF7zBFr
i2ESMVpW41P
0nT02crSAKn
XRmNA5fQmSB